Melanoma or cancer of the skin, while one of the most common of all cancers, is also often the most serious, with a high incidence of fatality. Melanoma is extremely tough to treat, especially after it metastasises. Metastasis is when melanoma cancer cells spread to other parts of the body and grow. Cutaneous or skin melanoma is the principal form of this disease; however, it can also manifest in other ways, such as uveal or mucosal melanoma.
Given the high incidence of mortality from melanoma, it becomes increasingly important to detect and identify this disease in a timely fashion so that treatments can be personalised and effective. While histology remains the backbone of melanoma diagnosis, recent advances in diagnostic molecular testing in labs are proving particularly effective when histologic outcomes are equivocal for patients at risk of developing stage 3 and 4 metastatic melanomas.
Besides diagnostic testing, laboratory testing with unequivocal results also aids in monitoring the condition and prognosis. Dermatopathologists perform several molecular testing techniques beneficial to patients selected for targeted gene therapy, genetic risk assessment and clinical trials.
Through our articles, Helvetica Health Care (HHC) aims to spread awareness about serious diseases and the latest technologies and treatments supported by our high-quality lab products. Herein, we inform you about melanoma and the importance of molecular testing in treating this condition.
What is melanoma?
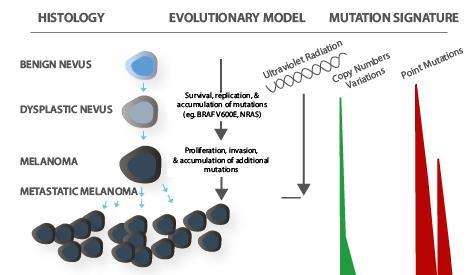
Melanoma is a type of skin cancer caused due to the malignancy of melanocytes or when melanocytes develop abnormally. Melanocytes are skin cells that produce melanin which gives the skin its pigmentation. External conditions, such as prolonged or intense exposure to UV radiation, genetic abnormalities resulting from a family history of melanoma, or the existence of particular polymorphisms (genetic mutations), could contribute to the development of this disease.
Based on symptoms experienced, several types of biopsies can be performed on lesions suspected to be malignant such as
- Skin or cutaneous biopsy
- Shave (tangential) biopsy
- Punch biopsy
- Excisional and incisional biopsies and
- “Optical” biopsies
The primary method for melanocytic lesion diagnosis is a histopathologic analysis of a skin biopsy. Yet histopathologic diagnosis has its limitations in achieving this goal, and, in particular clinical cases, molecular testing may be required to render a definitive diagnosis.
What is molecular testing?
Molecular testing is a lab procedure that uses a sample of tissue, blood, or another bodily fluid to look for specific genes, proteins, or other molecules that may indicate a disease or condition, like cancer. This method is also helpful in confirming and keeping track of certain chromosomal or gene alterations that can make a person more susceptible to cancer or other disorders.
Some cancer forms may be diagnosed using molecular testing in conjunction with other techniques, like biopsies. Additionally, it can generate a prognosis, determine how well the treatment works, and predict whether cancer will return or spread to other body regions, a method commonly known as molecular profiling and biomarker testing.
Why is molecular testing important?
Currently, primary cutaneous melanoma is diagnosed based on histology (tumour depth and ulceration) and clinical (number of lymph nodes and/or distant metastases) criteria. Sentinel lymph node biopsy does not detect all patients at risk for distant metastasis, which can happen in patients with thin melanomas. Rapid technological advancements in molecular pathology provide fresh clinical prospects for the identification of subclinical disease, the assessment of the propensity of the disease, the selection of effective and non-toxic treatments, providing personalised treatment regimens and the monitoring treatment outcomes.
Molecular testing is beneficial in assessing tumour biology. Genetic mutations implicated in tumour pathogenesis can be found in many melanomas. Given the lack or existence of a specific mutation, accurate identification of these mutations is required to segment patients for the purpose of targeted treatments and potential for clinical trials.
For instance, the most common mutated genes in melanoma, BRAF (a human gene that encodes a protein called B-Raf that can proliferate and divide cancerous cells), affects almost half of all melanoma cases. BRAF mutation testing is critical in patients with stage III melanoma with a high risk for recurrence. These molecular tests can identify the potential of future BRAF-directed therapy.
Molecular tests can also be considered for
- C-KIT (a type of receptor tyrosine kinase and a type of tumour marker) and
- NRAS (neuroblastoma ras viral oncogene homolog) gene mutations
that are present in 10-15% of mucosal and acral melanomas in patients with stage IV melanoma with disease recurrence or at presentation.
Molecular testing also assists ongoing targeted therapies and immunotherapies that treat melanoma tumours using the body’s natural defences.
What are the types of molecular methods for melanoma detection?
A sample of the patient’s melanoma tumour is most frequently used for molecular testing. The most comprehensive characterisation and genetic data are provided through next-generation sequencing (NGS). It is also possible to find genetic mutations and genomic aberrations in melanoma tumour samples using a variety of molecular assays utilising cytogenetic techniques like
- comparative genomic hybridisation,
- fluorescence in situ hybridisation and
- gene expression monitoring, or immunohistochemical staining.
These lab tests are helpful when histopathologic results are ambiguous.
Next-generation sequencing (NGS)
NGS is a massively parallel sequencing technology in DNA sequencing that captures a broader spectrum of mutations. NGS platforms perform sequencing of millions of small fragments of DNA in parallel providing high depth to deliver accurate data and insight into unexpected DNA variation. NGS can sequence an entire human genome within a single day and find mutations in thousands of target regions simultaneously. Hundreds of additional genes, including NRAS and C-KIT, including BRAF, are simultaneously detectable in melanoma tumour tissue by this incredibly sensitive assay. As a biomarker for immunotherapy response, some NGS procedures can also measure tumour mutational burden.
Comparative genomic hybridisation (CGH) / single-nucleotide polymorphism (SNP)
CGH is a molecular cytogenetic technique that can examine copy number variations throughout the genomic DNA in cells. The benefit of CGH/SNP arrays stems from their capacity to present a comprehensive picture of the genome. According to studies, if a clonal aberration is present in at least 30% to 40% of the lesion, a CGH can detect it accurately.
Fluorescence in situ hybridisation (FISH testing)
FISH is a molecular technique that allows for the direct viewing of particular genomic DNA segments using fluorescent DNA locus-specific probes. The test identifies complementary genomic DNA sequences on metaphase and/or interphase nuclei in tissue sections. Compared to CGH, FISH testing provides several advantages, such as identifying alterations in discrete subpopulations within a heterogeneous lesion, a quicker turnaround time, and a lower tissue requirement.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
qRT-PCR can identify a gene’s most prevalent mutations. When only one or a few genes are of interest, PCR testing, which specialises in modest numbers of gene targets, can provide faster turnaround times for results and is optimal for BRAF mutation analysis.
Immunohistochemistry screening (IHC)
Immunohistochemical staining is highly specific and sensitive for detecting V600E BRAF mutation in melanoma.
Melanoma can spread to other body parts rapidly, making it a fatal disease. Once biopsy outcomes are positive, the challenges faced by patients are very demanding. Certain treatments for advanced melanoma rely on molecular tumour markers, so dermatopathology and molecular biology labs must step up the molecular testing process.
Labs must have set protocols to track, manage and report mutation tests, stay up to date on the latest testing methods and be equipped with high-quality diagnostic tools, solutions and equipment that can significantly improve the detection of melanocytic lesions.
HHC is a Swiss leader in the supply of EXTERNAL RUN CONTROLS, NAT controls, panels and serology controls designed to validate your molecular testing and will create confidence and assure consistent molecular results. Our products comply with ISO15189:2012, an internationally recognised standard and a valuable resource for medical laboratories.
This leads to better patient safety and quality outcomes and confirms the competence of medical laboratories by customers, regulating authorities and accreditation bodies’ competence. Our NATtrol™ products are non-infectious, refrigerator stable and supplied in a liquid format, ready to use. They are open systems and can be used across different molecular testing platforms.
Call us now to find out more about our products and services and to help melanoma patients receive prompt as well as targeted treatments with clear test outputs.