As the COVID-19 pandemic spreads worldwide and develops new mutations, precise diagnostic tools are becoming increasingly important in managing and comprehending the disease’s epidemiology. The influence of COVID-19 on Molecular Diagnostics laboratories testing and research for non-COVID uses has been underappreciated but arguably even more crucial since the pandemic.
Lack of equipment, reagent capacity, and adequate clinical laboratory environmental protection, including personal protective equipment, decelerated the adoption of COVID-19 molecular diagnostics in the early stages of the pandemic, adding to the physical exhaustion of research facility professionals dealing with sudden massive and urgent testing demands.
A less well-known negative impact has been on non-COVID molecular biology research, such as molecular diagnostics. For the last year, non-COVID research has been impeded by the reallocation of equipment and employees to COVID-19 projects, supply chain interruptions, and social distancing measures, especially at university institutions. Healthcare clinical research has been one of the most seriously impacted areas.
Structure and mechanism of action/pathogenesis of SARS-CoV-2
Spike protein comprises two subunits, S1 and S2, which help the structural proteins guide viral attachment, fusion, and entrance. S1 represents the big receptor-binding domain (RBD), whereas S2 aids in the development of the spike protein’s stalk. The RBD on S1 interacts with the Angiotensin-converting enzyme 2 (ACE-2) receptor, allowing the virus to enter, and the Transmembrane protease serine 2 (TMPRSS2) receptor allows S protein priming, which in turn is essential for viral transmission and pathogenesis.
The most prevalent M protein has three transmembrane domains that assist in shape vision, in addition to the spike protein. E proteins are microscopic proteins that help the virus assemble and release. The N protein has two domains that can bind to viral RNA.
The fifth structural protein is seen in -coronaviruses, hemagglutinin-esterase (HE), identified lately. It functions as a hemagglutinin and attaches to sialic acid, allowing the virus to enter the cell via the S-protein. SARS-CoV-2 primarily affects the lungs’ airways and epithelial cells, causing significant epithelial and endothelial cell death.
Viral infection and replication in the body cause aggressive inflammation and acute lung injury, as well as the secretion of a cytokine storm consisting of interleukins (IL-1, IL-10, and IL-4), interferon (IFN-), IFN-produced protein (IP-10), and monocyte chemoattractant protein (MCP-1).
The three primary molecular diagnostic procedures available are:
- Chest computed tomography (CT Scans)
- Reverse transcriptase-polymerase chain reaction RT-PCR (gold standard)
- Lateral flow-based chromatographic strip
As a result, antibody-based assays and real-time RT-PCR are being employed in tandem to develop COVID-19 diagnostic procedures that are rapid, accurate, specific, and highly susceptible. On the other hand, specificity refers to an assay’s capacity to accurately identify persons who do not have the illness by decreasing cross-reactive responses. Accuracy is a phrase that encompasses both sensitivity and specificity and refers to the assay’s ability to distinguish between sick and healthy individuals accurately.
Molecular Test Diagnosis
Molecular tests that identify the prevalence of the RNA genome and antigen tests that indicate the presence of viral antigens, like the viral protein coating, are utilised to help in the diagnosis of COVID-19 during an active infection. Serological diagnostics (antibody testing) targeting an individual’s immunological response after the disease was also used during the pandemic.
COVID-19 positive persons who are: symptomatic and develop moderate (paucisymptomatic) to severe illness; asymptomatic (may not exhibit symptoms); and presymptomatic (infected individuals who may create signs following a positive test result) are identified using molecular testing.
Factors affecting test sensitivity and specificity
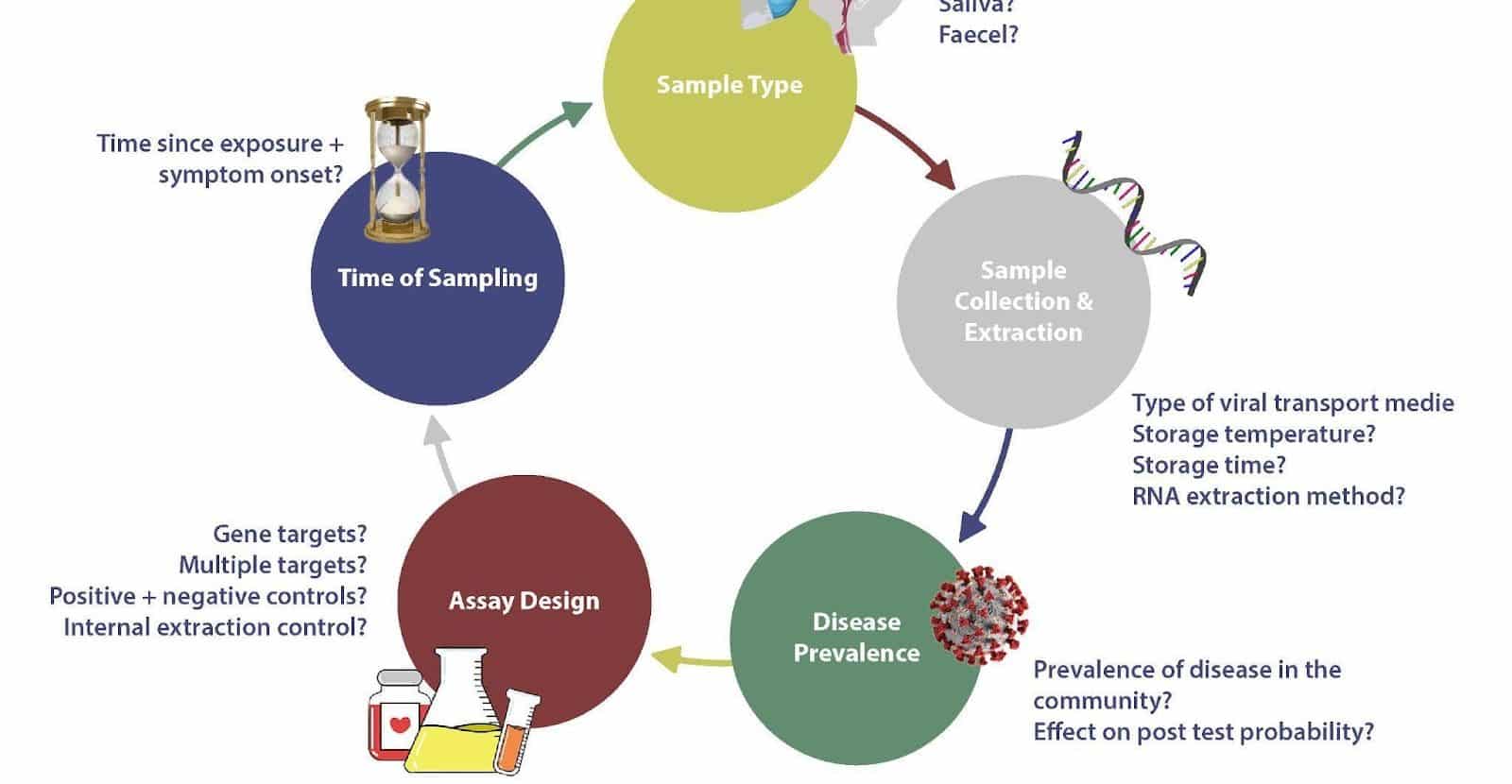
Several factors affect the accuracy of molecular tests, including the site and quality of sampling, the stage of disease, rate of viral clearance, and prevalence. In addition, design features of the molecular test are also essential to consider, e.g., gene expression targeted and the reliance on amplification from multiple targets. For these reasons, a test is never 100% accurate and the lack of a gold standard for benchmarking further performance compounds this problem.
Several variables affect test sensitivity and specificity.
The site and quality of sampling, the degree of illness, viral clearance rate, and frequency are all factors that influence the accuracy of molecular assays. Furthermore, you must consider molecular test design factors such as genes targeted and dependency on amplification from numerous targets. A test will never be 100 percent accurate for these reasons, and the lack of a gold standard against which to compare future performance exacerbates the situation. The elements listed can be used to generate false negatives and positives.
False negatives can result in infected people circulating in the population, unintentionally perpetuating the pandemic; insufficient sample loads can cause these false positives, sampling people too early or too late in the genetic disease cycle, or viral genome degradation.
False positives can arise due to reagent contamination, such as primer contamination or contamination during sample collection and processing. As a result, the number of SARS Cov cases may be overestimated. This section of the review will look at some of the other important parameters mentioned concerning the sensitivity of these molecular tests.
Demands for laboratory testing has increased since the Pandemic
The continuing SARS-CoV-2/COVID-19 pandemic has highlighted the use of molecular diagnostic technologies in the fight against infectious disease epidemics. Scientists and technicians have been developing diagnostics and laboratory medicines from the beginning of the epidemic.
Conclusion
It has been established that sensitive and specific molecular diagnostics assays for novel infections may be produced in months. On the other hand, the tests must be implemented in systems that allow for quick analysis and distribution of results to a wide range of decision-makers to be most successful.
This means that information systems must be streamlined and integrated, and the number of “seams in the system” must be significantly minimised. The COVID-19 experience has demonstrated what is required: molecular diagnostics at the point of care, with results reported on the same visit, simultaneous export of anonymised results to public health agencies and governments, and artificial intelligence techniques to help with test analysis and interpretation.
During the epidemic, mass manufacture of SARS-CoV-2 diagnostics helped overcome constraints associated with PCR product-based detection and increased testing capacity. Nonetheless, the development of these tests in s been complex, with issues relating to clinical accuracy, regulatory review, availability, and adoption in various healthcare settings throughout the world. Only time will tell how healthcare services, governments, and regulatory agencies throughout the world will adjust to changing requirements and the pandemic’s long-term consequences.
Visit the Helvetica Health Care website for more information on how our EXTERNAL RUN CONTROLS, NAT controls, panels and serology controls are designed to validate your molecular testing.